
Aluminum and aluminum alloys are among the most used metals in fabrication, construction, and in the creation of good all around you. It is such a useful and versatile metal, but there are some special considerations you need to make when working with it. We have put together this expert guide to fabricating, grinding, and finishing aluminum to make sure you are using your aluminum to the best of its abilities.
We'll start off with some helpful information and background on aluminum, which should provide some background on why it should be treated different than other metals like steel. Then we cover some of the best practices and what are the best abrasives for aluminum.
Skip Ahead:
- About Aluminum
- Why and where should you use aluminum?
- How much does an aluminum fabrication cost compared to steel and stainless steel?
- Can you improve the corrosion resistance of your aluminum fabrication?
- What forms or stock shapes of aluminum are available?
- If aluminum is softer and weaker than alloy steel, why use aluminum?
- What grades or types of aluminum are there?
- What does aluminum “temper” mean?
- What aluminum grade should I fabricate with?
- Forming Aluminum Alloys
- Welding Aluminum and Aluminum Alloys
- How do abrasives enhance aluminum welding and fabrication?
- Proper Abrasives and Cutting Tool Selection
About Aluminum
Why and where should you use aluminum?

Aluminum is used for several unique properties compared to steel:
- Lightweight –
- Aluminum has a density of 2.7g/cm3 (0.0975 lb/in³), which is about 1/3 of steel’s density
- Steel has a density of 7.85 g/cm3 (0.284 lb/in³)
- Good corrosion resistance
- High thermal and electrical conductivity
- High reflectivity
- Lower melting point – easier to cast or diecast
- Lower softening point – easier to extrude into complex shapes
- Weldable – but as not easily as steel
- High formability
- High machinability - softer and easier to cut
- Good grindability with proper abrasive selection
Outdoor furniture, boat hulls, aircraft parts, containers, and architectural elements like window frames and gutters are made from aluminum.
Aluminum is used to make heat sinks and radiators because of the metal’s high thermal conductivity or ability to transfer or dissipate heat rapidly.
Electrical wiring and conductors are made of aluminum especially in larger thicknesses because aluminum has high electrical conductivity (60% of copper’s conductivity) combined with lower cost and density compared to copper.
Aluminum has many advantages over steel, copper, and stainless steel, but there are also some drawbacks to fabricating with aluminum.
*The photograph in Figure 1 above is the cast aluminum cap on top of The Washington Monument.
Photo Source - Library of Congress
How much does an aluminum fabrication cost compared to steel and stainless steel?
The unit cost or cost per pound of aluminum is typically higher than the cost of carbon or alloy steel, but much less than stainless steel. However, since aluminum is much lighter or lower in density compared to stainless steel or carbon steel, an aluminum part of the same dimensions can cost less.
The cost-effectiveness of aluminum is why beer and soda cans are made of aluminum instead of steel. Aluminum fabrication costs can be lower compared to other metals because aluminum is very formable, and machinable.
Can you improve the corrosion resistance of your aluminum fabrication?
Aluminum has much better corrosion resistance compared to carbon or alloy steel, but not as good as stainless in extreme environments. If your fabrication project is going to be sitting in a dry, indoor location, then you probably will not need to worry about corrosion of your aluminum project.
For example, think about your aluminum, cast iron, steel, and stainless pans in your kitchen. A cast iron or steel wok will rust quickly if not protected with oil or a baked-on carbonized film. Your stainless steel pans will wear out before they show signs of corrosion. Your aluminum pots will last a long time before showing signs of corrosion. Exposure of aluminum to salty brines or acids will accelerate corrosion. Non-stick coatings and exterior coatings on aluminum pans enhance corrosion protection.
A layer of paint or an epoxy coating on an aluminum boat part or outdoor furniture will protect against corrosion. If you are making a tool or gage for your shop, then you probably do not need an additional coating if your shop is dry.
A protective top coating on aluminum will prevent corrosion on outdoor projects, but not after the coating chips, delaminates, or is worn off the surface.
The corrosion resistance of aluminum fabrications can be enhanced by:
- Passivating the aluminum surface
- Chromate conversion coating
- Anodizing the aluminum surface
- Painting the aluminum surface
- Powder Coating
Passivating Aluminum
Aluminum can be passivated like stainless steel.
Passivation involves the removal of any surface impurities and restoration of a passive oxide film or other protective films. The passive oxide film prevents corrosion by creating an inert, non-porous barrier. Chemical treatment, conversion coatings, and anodization can passivate aluminum surfaces.
Aluminum can be passivated through repeated immersions in nitric acid-peroxide and deionized water baths, which removes harmful impurities and builds a passive oxide film. The more corrosion-resistant aluminum alloys can auto-passivate or naturally develop a passive oxide film in the air. Less corrosion-resistant alloy like the 2xxx series alloy (2024) required passivation or protective coatings.
Chromate Conversion Coating Aluminum
Chromate conversion coating is another method to passivate an aluminum surface. Chromate conversion is accomplished using chromic acid or chromic acid combined with fluoride salts. Alodine, Iridite, Bonderite, and Alocrom are well-known brands names for aluminum conversion coatings.
Aluminum parts are brushed with, sprayed with, or dipped into the conversion coating solution to form a yellowish or golden brown film with a thickness of 0.00001–0.00004 inches. The conversion coating process is very quick taking only 1 to 5 minutes. The excess chromate solution is wiped off and parts rinsed.
Anodizing Aluminum
Anodization passivates aluminum by creating a thicker hydrated aluminum oxide film.
The aluminum oxide film that forms naturally in the air on aluminum alloys is not a coherent and non-porous as the chromium oxide film on stainless steel. Anodization is an electrochemical direct current (DC) process where aluminum is the anode in an electrolytic cell. The electrolyte solutions employed are typically acids such as chromic acid for type 1 anodization or sulfuric acid for type II anodization. Hard coat or type III anodized aluminum uses a longer temperature, longer treatment time process to grow very thick and wear-resistant layers.
The acids dissolve the aluminum oxide film while the oxidation rate is increased through the application of DC power. The result is the growth of an aluminum oxide film with nanopores, 10–150 nm in diameter. The nanopores allow the alumina layer to continue to grow to much greater thicknesses compared to oxide films formed in the air. Anodized aluminum is not only more corrosion-resistant but also more wear-resistant. Hard anodized aluminum surfaces are optimized for wear resistance.
Dyes can also be applied to color the anodized layer. The dyes can penetrate the aluminum oxide pores. After anodizing treatment and dye application, the anodized aluminum oxide layer is sealed by converting the aluminum oxide layer to aluminum monohydrate. The hydration process swells the oxide layer to fill and close the pores.
Paint or Powder Coat Aluminum
Protective paints and powder coating can be applied to an aluminum surface to provide additional protection. The aluminum surface should be chromate conversion coated before painting to optimize corrosion protection.
The aesthetics or beauty of aluminum is another reason to use the metal in your fabrication projects. With the proper selection of abrasives for surface preparation and surface treatment or coating, a wide variety of gorgeous finishes can be generated on aluminum.
The correct series of abrasive can prepare an aluminum surface for conversion coating, anodizing, powder coating, or painting.
The assembly process can also deteriorate the corrosion resistance of aluminum. If your aluminum parts are bolted together using copper-based brass or bronze fasteners, then the areas under the nuts, washers, and bolt heads will likely show signs of galvanic oxygen in damp or wet environments. galvanic corrosion occurs when more noble metals like copper or stainless steel are coupled to less noble metals like aluminum, zinc, and carbon steel.
What forms or stock shapes of aluminum are available?

Figure 2 - Manufacturing aluminum shapes - bar, rod, sheet, plate, tubes, ingots, wire, extrusions, forgings, and castings
Source - EPA.gov
Another advantage of aluminum in fabrication is the wide availability of shapes and processing methods. Since aluminum has a much lower melting and softening point compared to steel, the metal, and its alloys can be easily formed into shapes by extrusion, forging, casting, rolling, and drawing.
Aluminum extruders often have catalogs of common shapes, which can have complex configurations. Aluminum extrusions with the proper shape might eliminate the need for welding or fastening several parts together to fabricate your aluminum design or project. Specialized extruded aluminum shapes are available for sailboat masts, T-slot framing, window parts, structural profiles, door parts, and finned profiles for heat sinks.
The low melting point of aluminum makes backyard metal casting more feasible compared to steel or stainless steel. Care should be taken when metal casting to make sure the mold or investment materials are baked out and dry to avoid explosions. Die casting can produce extremely complex parts to tight tolerances, but the cost of the steel molds is prohibitive for small runs.
Aluminum is softer and weaker than alloy steel, so why use aluminum?
Certain aluminum alloys can exceed the tensile and yield strength of carbon steel. For example, 7075 aluminum has a yield strength of 73,000 psi, and carbon steel has a yield strength of 47,900 psi. Yet, aluminum has 1/3 the density of carbon steel. UTS or ultimate tensile strength is the stress or pounds per square inch where an alloy will fail or break. YS or yield strength is the stress where the metal will start to permanently deform. We typically design with yield strength. A metal’s ductility is given by the % elongation or how much the metal can stretch or deform before it breaks.
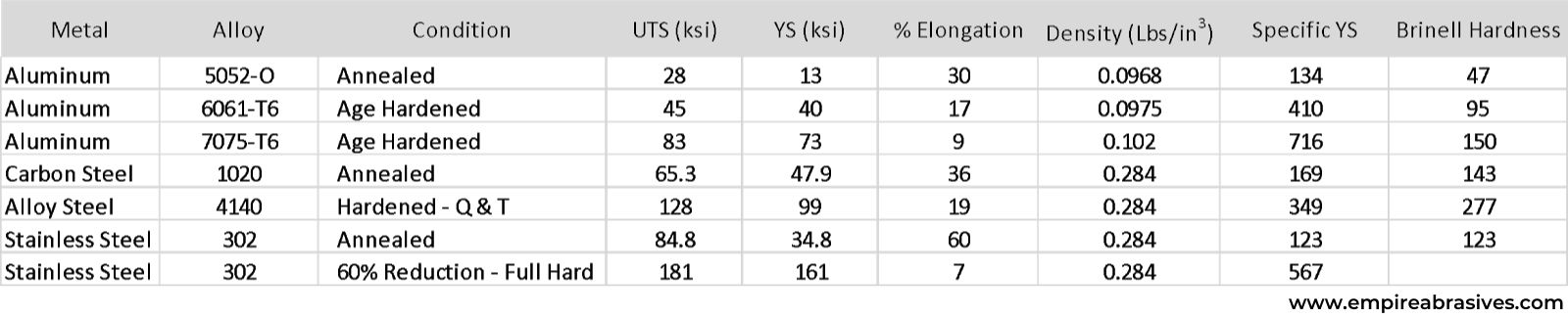
While many aluminum grades are softer and weaker than heat-treated alloy steels, aluminum can still be advantageous for structural applications such as aircraft and boat structures because the strength-to-weight or specific strength of an aluminum alloy can be higher than some alloy steels.
Specific strength is calculated by dividing the alloy’s strength by its density. While 6061-T6’s yield strength of 40 ksi or 40,000 psi is much lower than hardened 4140 alloy steel’s 99 ksi yield strength, the specific yield strength or strength-to-weight ratio of 6061-T6 is 410 compared to 349 for 4140 alloy steel.
Aluminum is a great choice for many projects where you want the strength of metal combined with lightweight properties. An aluminum chair, bike frame, or cart will be much lighter and easier to pick and move compared to a steel version of the same thickness and dimensions. The high specific yield strength can be fully utilized in projects or designs where the stress can be distributed. If the stress is highly concentrated such as a latch or hinge, then a hardened or stronger steel or stainless steel alloy might be a better choice.
What grades or types of aluminum are there?
As with carbon, alloy, and stainless steels, there are many different grades or types of aluminum depending on the specific ingredients or alloying elements.
The designation or numbering system for aluminum alloys was developed by the Aluminum Association. Aluminum can be grouped into major families based on the main alloying element used to strengthen or modify the aluminum alloy.

1xxx Series Aluminum
The first number in an alloy number designation indicates the family. 1000 series or 1xxx series alloys are the groups of pure aluminum grades. 1xxx grade contains only minor amounts of other metals. The high purity of the 1xxx series results in high electrical and thermal conductivity as well as low strength and high formability. As a result, these alloys are commonly used to make electrical aluminum conductors, packaging foil, food trays, capacitors, reflectors, and heat exchangers.
2xxx Series Aluminum
The 2xxx series are strengthened with copper additions, which allow the 2xxx alloys to be heat treated. The downside of copper additions is poor corrosion resistance. 2xxx series alloys need to be anodized or coated for exterior or corrosion-prone environments. Aircraft parts, automotive components, and other structural products requiring high strength and lightweight are often made from 2xxx series alloys because the 2xxx series aluminum can be heat treated to high strength levels.
3xxx & 4xxx Series Aluminum
3xxx series aluminum uses manganese additions. 3003 aluminum is a widely-used general-purpose alloy. 4xxx series contain silicon and are mainly used as filler alloys and for automotive pistons.
5xxx Aluminum
5xxx series aluminum alloys contain magnesium. 5xxx alloys are very corrosion-resistant and weldable. They are often considered marine-grade aluminum alloys. They are widely used in boat hull building.
6xxx Series Aluminum
6xxx series aluminum alloys use a combination of magnesium and silicon additions to create heat-treatable alloys. 6xxx series alloys have a combination of good strength combined with better corrosion resistance than 2xxx series alloys.
7xxx Series Aluminum
7xxx series aluminum use zinc in combination with other elements to enable precipitation hardening to the highest strength levels. However, the corrosion resistance is poor, so coating or anodizing is required to protect the metal surfaces in wet or corrosive applications.
The aluminum alloy series can be split into two groups:
- Non-heat treatable series – 1xxx, 2xxx, 3xxx, 4xxx, 5xxx
- Heat treatable series - 2xxx, 6xxx, and 7xxx alloys
8xxx Series Aluminum
The 8xxx series of alloys contain specialized, miscellaneous alloys of aluminum with iron, lithium, and other elements for electrical conductors and aircraft components. Some alloys are work-hardened while others are heat treatable.
Heat treatable aluminum alloys are hardened and strengthened by precipitation hardening. In the precipitation hardening process, the alloy elements such as copper or zinc are dissolved at an elevated “solution treatment” temperature. The aluminum alloy is then hardened by precipitating out very fine particles at a lower “aging” temperature. For example, some 2xxx aluminum alloys designed for aluminum riveting are kept refrigerated and then age harden at room temperature after assembly in an aircraft.
The non-heat-treatable aluminum alloys are strengthened by work hardening or mechanical deformation during rolling or forming. The degree of work hardening or deformation is designated by ¼ hard, ½ hard, ¾ hard, full hard, or extra hard.
What does aluminum “temper” mean?
Aluminum alloys are typically supplied in forms with both a grade and temper designation. The aluminum alloy's temper describes the alloy’s condition in regards to thermal processing, degree of work hardening, or heat treatment.
The temper or condition of the aluminum alloy is described after the grade with a letter followed by the number: grade-temper – for example 6061-T6.The temper letters mean the following:
- An F temper aluminum alloy is in the as-fabricated condition after extrusion, forging, rolling, etc.
- An O temper aluminum is in the annealed or soft condition.
- H tempers apply to non-treatable alloy strengthened by work hardening as we do with non-hardenable 300 series stainless steels.
- W tempers are in the as solution treated condition
- T tempers apply to heat-treatable alloys and indicate the aluminum is in the solution treated and aged condition. Aging could be artificial or natural.
A 5052 O alloy would have a yield strength of 13,000 psi while a 5052 H aluminum alloy ranges from 28,000 psi (H32 temper – ¼ hard) to 37,000 psi (H38 temper - full hard). If you are going to cut, form, and weld 5052 aluminum shapes into your assembly project, then you might as well use 5052-O temper aluminum because the welding heat will anneal aluminum to properties equivalent to the O temper or annealed condition.
A T6 temper would indicate that the aluminum alloy was solution treated and artificially aged to precipitation harden and strengthen the alloy. A 6061-O aluminum bar would be softer and lower in yield strength (8,000 psi) compared to a 6061-T6 aluminum bar (40,000 psi). 6061-T4 aluminum is solution-treated and naturally aged, which results in a yield strength of 21,000 psi.
If you are going to cut, form, and weld aluminum shapes into your assembly project, then using a harder 6061 in the T6 condition might not be much better than 6061 T4 aluminum because the metal in the weld or weld heat affect zone (HAZ) will be softened by the welding heat. The weld strength of heat treatable 6061 aluminum is about 15,000 psi, which is slightly less than a T4 temper or solution treated condition.
You should design your project with the understanding that the arc welds will be weaker than the surrounding metal when using age hardened 2xxx, 6xxx, and 7xxx alloys.
What aluminum grade should I fabricate with?
While there are hundreds of standard industrial alloys and many proprietary alloys to choose from, the most commonly available and used aluminum alloys include 6061, 2024, 5052, 3003, 1100, and 7075.

Many of the other aluminum alloys are based on these “workhorse” alloys with the composition and processing tailored for specific applications. You are better off sticking with one of the common “workhorse” or mainstream alloys for a project because these metal alloys will be easier to find and lower in cost since larger quantities are routinely produced daily. Proprietary and specialty alloys can be difficult to procure and costly.
Aluminum alloy selection for:
- Corrosion Resistance - 6061, 5052, 3003, and 1100 should be suitable for industrial and marine applications without any additional protective coatings. 5052, 3003, and 1100 will have superior corrosion resistance. Alclad aluminum alloy consists of 2024 with an outer later of 1100 aluminum to boost corrosion resistance while allowing utilization of age-hardened strength levels of 2024. 6061 might provide sufficient corrosion resistance with age-hardened strength.
- Formability – Most of the common aluminum alloys have good to excellent formability except for the remarkably high strength age-hardened 7075-T6 alloy.
- Machinability – The softer aluminum alloy form longer continuous chips resulting in poorer surface finishes and reduced machinability. Longer aluminum chips tend to load up the teeth of saws and cutters. The age or work-hardened alloys generate smaller chips during cutting and are easier to machine because the chips can clear the cutting zone.
- Arc Weldability - The work hardening grades such as 5052, 3003, and 1100 have excellent arc weldability using MIG (GMAW) or TIG (GTAW) arc welding processes. The weld strength of work hardening grades will only be as strong as the annealed or “O” temper of the alloy. 2024 and 7075 have poor to moderate weldability. 6061 is more weldable than 2024 and 7075, but the weld strength will be reduced to a solution treated or T4 temper strength levels.
Forming Aluminum Alloys
Is aluminum as formable as carbon steel?
Aluminum is softer and more ductile compared to many carbon and alloy steels. As a result, aluminum alloys are typically easier to form compared to many steel alloys – especially in the O or annealed condition. Cold worked or H temper aluminum alloys will of course be more difficult to form because ductility decreases with the cold work. An Hx2 (1/4 hard) temper aluminum will be more formable than an Hx8 (Full Hard) temper.

Like steel and stainless steel, aluminum alloys have forming limits. If the minimum bend radius for the alloy is exceeded, then cracking can occur on the outside edge of the bend. The higher strength heat-treated alloys tend to require a larger radius to prevent cracking. Bending the part with the grain or rolling direction versus across the rolling direction can also impact the minimum bend radius. You will have to make a larger bend radius if you are bending with the grain (longitudinal) versus across the grain (transverse).
If you have work-hardened an aluminum part to the point where any additional forming will crack the part, then the aluminum part can be annealed to allow additional forming. Aluminum can be annealed and hot-formed at much lower temperatures compared to steel alloys. While more complex shapes can be hot formed, annealing and hot forming will alter the temper of the alloy and reduce strength.
Welding Aluminum and Aluminum Alloys
Is aluminum more difficult to weld compared to steel?
Once your aluminum parts are formed, they may need to be welded. An inert gas or gas mixture is required if you are going to MIG or TIG weld. Argon gas protects the aluminum the weld pool and surrounding base metal. Argon inert gas prevents excessive aluminum oxide formation and a “black soot” or “smut” appearance.
- Aluminum’s high thermal conductivity sucks the heat away from the weld – so you need to weld aluminum fast and hot – a higher power or a more concentrated heat source compared to steel or stainless steel.
- Aluminum has a much lower melting point of 1221°F compared to steel’s melting point of 2600°F, so it is much easier to burn through aluminum during welding, which is why you need to keep the arc moving across the joint quickly.
- Aluminum parts have an oxide film, which needs to be removed for high-quality welds.
- The oxide film reforms after welding and during welding on unshielded aluminum surfaces such as the back of welds. Poorly shielded aluminum welds typically have a smut or black sooty appearance.
To create watertight welds such as those required on a boat hull, the backs of the aluminum welds should be ground or cut with a carbide tooth saw blade or rotary cutter to remove the aluminum oxide film before a second weld pass is laid down on the backside of the weld joint.
The proper cleaning and surface preparation of the aluminum joint surface is important in forming good welds in aluminum. Stainless steel wire brush, chemical etching, or abrasive cleaning should be employed if the aluminum has a thicker oxide layer.
How do abrasives enhance aluminum welding and fabrication?
Abrasives can be used to enhance several steps in the welding and fabrication process:
- Cutting aluminum
- Deburring cut edges
- Preparing aluminum surfaces for welding
- Back cutting and back grinding welds
- Cleaning and blending aluminum welds
- Polishing to a brushed or satin finish
- Buffing to a polished or mirror finish
Cutting Aluminum
Aluminum sheets and plates can be cut into intricate 2D patterns using a water jet or rotary cutting tables.
Aluminum is much softer than steel, you can even cut aluminum with carbide-tipped woodworking saw blades and tools. However, there are router bits, carbide burrs, rotary saw blades, and other cutting tools designed specifically for cutting aluminum.
Bits for aluminum tend to have larger teeth with an open face or burr spacing to allow fast chip or swarf removal. A rotary cutter designed for steel will often load quickly with aluminum and become ineffective. Carbide rotary burrs designed for cutting aluminum and soft metals are called “aluminum cut” or nonferrous burrs.
If you are just cutting simple shapes from stock like sheet, plate, tube, or, bar, then you have several options:
- Tin snips or hand shears can be used to cut thinner aluminum sheet.
- Power nibblers and power shear tools can cut slightly thicker aluminum sheet.
- Metal shear presses can quickly shear off a large sheet or make a long straight cut across a thicker aluminum sheet or plate. A shear capable of cutting ¼ inch carbon steel sheet will be able to cut a very thick aluminum sheet.
- Toothed saw blades mounted on band saws, reciprocating saws, or circular saws can cut bar, rod, tube, plate, and sheet.
- Abrasive cutting wheels mounted in chop saws, circular saws, and angle grinders are useful in cutting cut bar, rod, tube, and narrow plate.
If toothed saws are used to cut aluminum, then they should be carbide-tipped saw blades (tungsten carbide teeth). Bi-metal high-speed steel-carbon steel blades may cut aluminum too, but they will not have the life and the straight cutting of carbide-tipped blades. Most aluminum alloys will saw cut faster than carbon or alloy steels.
Holes are drilled or cut using drill bits or hole saws mounted in drill presses or power hand drills. Holes saws work well on sheet metal and thinner aluminum plate. Drill bits are a better choice for thicker stock.
Ideally, cutting lubricant or coolants should be used when toothed saw cutting or drilling aluminum. They will reduce friction and help cool the parts minimize heat generation and softening of the alloy. Unlike steel alloys, active sulfur and chlorinated machining fluids are not useful. Simple mineral oils or WD-40 will work fine in reducing friction and the sticking of aluminum to the cutting teeth.
While you can use a toothed carbide blade to cut aluminum, an abrasive cutoff wheel can cut aluminum very quickly. With thinner aluminum sheet, tin snips or carbide-tipped saws are fine. When cutting thicker aluminum bar, rod, angle iron, and other structural shapes, a cutoff wheel in a chop saw might be the way to go.
Apply steady, moderate pressure when cutting aluminum with an abrasive wheel. Excessive pressure will generate more heat, which will soften the aluminum. Softened aluminum will be gummier and can result in loading of the abrasive cutting edge with aluminum. Loading is a common problem when cutting and grinding aluminum. When loading occurs, the abrasive is covered with aluminum and will no longer cut. Cutoff wheels designed for aluminum cutting use a blend of silicon carbide and aluminum and other proprietary ingredients to prevent loading.
If you are using a cutoff wheel in an angle grinder, then be careful not to twist or bend the blade while cutting. Cutoff and grinding wheels can be broken this way. Thinner cutting wheel blades tend to cut cooler with minimum kerf.
Deburring Aluminum
Shearing, saw cutting, and abrasive cutoff wheels typically leave a sharp burr on the cut aluminum edges. Drilled or saw cut holes also tend to have burrs as well.
Why is deburring your parts important?
While aluminum is softer than steel, sharp aluminum burrs can still give your fingers and hands nasty cuts when you grab the cut edge of a part, so always take a minute to remove the burr on your aluminum edges.
Coated abrasives (belts, discs, or flap discs), nonwoven abrasives, metal files, deburring blades, and wire brushes can be used to deburr aluminum parts.
There are metal files and deburring blades designed specifically for deburring aluminum and soft metals. The teeth on the files and blades have different shapes or cutting angles compared to blades for deburring steel or plastic, which reduces clogging or loading.
Aluminum is softer than steel, the abrasive tips can dig in more easily creating thicker chips. The high ductility and low annealing or softening temperatures of aluminum result in a tendency to form long chips and gumming up or loading of abrasive surfaces. Try to grind aluminum gently with light pressure and let the abrasive do the work. Bearing down or applying high pressure during aluminum grinding may result in premature loading of the abrasive surface.
Pre-cleaning and Preparing Aluminum Surfaces for Welding
Like any weld joint, the surface needs to be cleaned and prepared before welding. However, aluminum is less forgiving than carbon steel in terms of contamination from dirt, films, grease, and oil. MIG and TIG welding requires an inert or protective shielding gas. Contaminants can disturb the shielding gas and lead to discoloration in the weld or surrounding heat-affected zone.
There are several different options for removing the oxide layer on aluminum before welding:
- Dipping or brushing parts with a weld prep solution. Some of these chemical cleaning methods use hydrofluoric acid to dissolve the aluminum oxide layer. You should read and understand the Safety Data Sheet (SDS) before using these chemicals.
- Stainless steel wire brushes – Some welders will not use abrasive products for weld joint surface preparation because they believe abrasive grits may get trapped in the soft aluminum resulting in a weld defect.
- Abrasive wheels, flap discs, nonwovens, or specialty coated abrasives – Silicon carbide disc should be able to remove any oxide layer without trapping any harmful grit in the weld.
Abrasive grinding wheels are also important in preparing the proper fit between the parts in the joint. On larger thicknesses, the aluminum pieces need to be beveled with cutting or grinding. If multiple passes are used, then grinding between passes to remove oxide layers or contamination. The backside of root passes of aluminum welds on boat hulls are normally cut with carbide cutters or carbide tipped saw blades before a second weld pass is applied on the backside.
The aluminum should be degreased before welding with acetone or an alkaline detergent cleaning solution. Alcohol should not be used to clean or degrease aluminum because it can react with the metal. The metal should of course be dry. Moisture can introduce hydrogen, which can cause porosity in aluminum welds.
Aluminum Weld Post Cleaning and Treatment
Abrasives are an essential tool for post-treatment and cleaning of the weld and the surrounding metal.
Pickling paste, electrochemical weld cleaners, pickling baths, abrasive blasting, nonwoven abrasives, stainless steel wire brushes, and coated abrasives are typically used to remove any discoloration or black soot or smut on the aluminum weld and surrounding heat effect zone.
The acid pickling baths and pastes contain phosphoric acids or a combination of hydrofluoric and nitric acids, which are toxic and require special measures to handle safely. Abrasive cleaning is safer and just as effective. Electrochemical weld cleaners are costly and require chemicals. In industry, a combination of abrasive grinding followed by pickling is often employed.
Coated abrasive, nonwoven abrasive, and stainless steel brushes will remove the smut and oxide layers remaining on welds. Even a cut buffing step might be aggressive enough for post-weld cleanup of aluminum joints. Avoid removing a lot of material outside the weld bead because this reduces section thickness and therefore strength.
Aluminum oxide scale in the root or backside of aluminum welds typically forms if the back is not protected with a purging gas. Depending on the furnace atmosphere, oxide scales are also formed on aluminum during heat treatment and forging. If a second pass backside weld is going to be made, then carbide tipped saw blades, carbide rotary cutters, and abrasive products are required to remove the oxide scale.
Slag, weld spatter, undercuts, cracks, pores, and other aluminum steel welding defects also need to be removed with abrasives or carbide burrs.
Weld bead blending, take down, or flattening can be done with a 50, 60, 80, or 100 grit abrasive disc, flap disc, or belt. Weld defects could also be removed with 50 to 80 grit abrasives.
Try leveling weld or parting lines with an 80-grit abrasive product first. If metal removal is too slow, then go to coarser grits. Changing abrasive product types instead of using a coarser grit size is a better approach.
If 80 grit size aluminum oxide abrasive product isn’t cutting it, then switch to a supersize zirconia or supersized ceramic product. These products will remove material faster without generating the deep scratches or digs caused by grinding with coarser 50 or 40 grit abrasives.
If your welds are well made and mostly flush, then a 100 or 120 grit flap disc could be sufficient to flatten and blend your welds.
While a coarser grit size can remove material faster, there are several reasons to use a finer grit abrasive when leveling and blending aluminum welds:
- Aluminum is much softer than alloy steels and stainless steels, so coarser grit abrasives can dig or gouge more easily.
- Coarser grits tend to generate more friction and therefore more heat – heat can cause distortion, residual stress, and even annealing or softening of the aluminum on certain tempers
- With the aggressiveness of coarser grit, it is easier to thin the metal surrounding your aluminum weld. Thinning the base metal reduces strength.
- Chances are if you are using aluminum, then you will want a satin, anodized, or painted appearance in your final product. Coarser grits leave behind a rougher surface requiring more work and abrasive steps to remove deep scratches and generate the final required finish.
- Removal of coarse grit scratches is also important for maintaining corrosion resistance. The valley or pit of a scratch gets less oxygen and makes the metal more susceptible to crevice and pitting corrosion.
Finishing Aluminum
After the welds have been leveled and blended, then the required surface finish is generated in a multiple-step process.
Setting the Grain
Graining or setting the grain is the first step in generating a surface finish on aluminum. Graining is developing a unidirectional scratch pattern across a metal’s surface. This step is also known as pre-polishing, polishing, or brush finishing.
For some applications, a grained or brushed surface finish might be fine and even more desirable. Brushed metal surfaces do not show fingerprints and smudges as much as mirror-polished surfaces. If you mirror polish your project, then you will be spending time repeatedly cleaning it later.
Coated abrasive belts, drums, or disc polishing is different than producing a polished surface by buffing. Non-woven abrasives can also be used for graining, brush finishing, or pre-polishing.
Using a progressively finer series of coated abrasives can reduce the final buffing time and produce a better finish. A series of coated abrasive steps could include:
- Pre-polishing with 80 to 120 grit – your blending step after welding may have already done this.
- Polishing with 150 to 240 grit
- Final or fine polishing with 280 to 400 grit
The specific series of abrasives can vary depending on the initial roughness of your aluminum project. You need to try a few different abrasive grit sizes and products to determine what is best for your application.
If you used 80 grit or 100 grit to flatten and blend welds, then you might be able to skip to the fine polishing step using 280 grit. A combination of 120 grit coated abrasive followed up with medium and fine nonwoven surface conditioning abrasives is another option.
With nonwoven abrasives, you could use medium, fine, and ultrafine products to polish before buffing.
If you are using a nonwoven or coated abrasive belt or drum to produce a unidirectional grain or grit line pattern, then each progressive polishing step should be done at a 90-degree angle or against the grain of the last step. This will help you recognize that you have removed the scratch pattern from the previous step. Sometimes the shape of the project or assembly may not allow this.
A brushed finish is generated with 150 to 240 coated abrasive belts or drums or medium very fine nonwoven abrasives.
A matte or satin finish is generated with a 220 to 240 grit coated abrasive or very fine nonwoven abrasive belt, drum, or disc.
Mirror Finish versus Grain Finishes on Aluminum
How do you get a mirror finish on aluminum? The short answer is through cut buffing and color buffing. These steps are used to produce a final polished or mirror finish after the initial refinement steps. An earlier article, “How to Polish Aluminum - A Beginner’s Guide”, provide details on the steps to generate a mirror-polished aluminum surface.
The question you should be asking is, Do I really need a mirror-finished aluminum surface?
In most applications, no because aluminum is so soft the polished surfaces will scratch quickly in applications where the project is handled or touched. If there is dirt, sand, or hard particles, the mirror finish will dull so after being put into use. You could try protecting the polished aluminum surface will an application of a clear topcoat or acrylic plastic sheet. If you are making an aluminum reflector for a lighting or solar concentrator project, then a polished aluminum surface could be useful. Just design the project so the aluminum surface is protected or difficult to handle.
In most applications, aluminum with a grained surface, a surface smoothed through surface conditioning with a nonwoven, and a coated abrasive unidirectionally sanded surface will be fine especially if the aluminum parts are going to be anodized or painted. Some roughness on the surface will allow coatings more surface area and undercuts to grab onto, which increases coating adhesion. Anodizing is going to dull the surface because oxidation and roughening occur in the process. An anodized surface will be two to three times rougher than the pre-anodized surface. Buffed or exceptionally smooth aluminum surfaces before anodizing will result in smoother, matte anodized surfaces.
Fastening, Mill Finishes, and Touch-up Finishing
While we have assumed your aluminum project was fabricated by cutting, forming, and welding. Mechanical fastening is another option for assembling your project. If you use bolts, nuts, washers, or other fasteners in a marine or corrosion-prone place, then make sure the fasteners are made of the same aluminum alloy grade as the sheet metal, plate, or bar stock. This will reduce the chance of galvanic corrosion of the fasteners. Avoid fastening aluminum with brass, steel, and galvanized steel fasteners because in wet environments these dissimilar metal combinations will cause galvanic corrosion.
Stainless steel fasteners will work in joining aluminum in most cases. In wet salty seawater or corrosive industrial environments, you may need to take an additional step of isolating the dissimilar metals with plastic washers, protective coating or paint, and sealants. Prep the area around the hole for painting and coating and then apply a protective coating to the aluminum before fastening.
A fastened assembly can open up new finish possibilities. A wide variety of mill finishes are available for aluminum sheet and plates. For example, tread plate aluminum sheet has an embossed diamond pattern and is commonly used for truck toolboxes, deck surfaces, and steps. Once you cut and drill holes on a mill finished sheet, you will need to deburr and touch up around the drilled hole or cut edges. Touch-up finishing from handling and end-use might be needed on a welded project as well.
Quick change miniature coated abrasive and nonwoven discs can be a good option for deburring and touching up around drilled holes or removing scrape marks. 2" Quick Change Surface Conditioning Discs and 2" Quick Change Aluminum Oxide Discs are good to have on hand for aluminum touch-up jobs.
Cross-contamination with Steel
Transfer of steel or iron onto aluminum can occur from steel parts and used abrasive products.
Avoid any cross-contamination of your aluminum parts with steel or iron. The steel residue left on the aluminum surface can corrode and leave rust stains. If steel parts or tools (punches, hammers, etc.) from other projects rub against your aluminum part, then they might transfer some steel to the surface, which will degrade corrosion resistance.
Sources of iron contamination to avoid include:
- Steel or iron dust from grinding carbon or alloy steel parts
- Abrasives used to grind carbon or alloy steel parts
- Steel brushes or carbon steel wool
- Use stainless steel brushes and stainless steel wool
- Steel blasting shot, grit, and tumbling media
- Vises, clamps, chains, or holders
- Carbon steel tools – hammer, screwdrivers, chisels, etc.
- Forming rolls, molds, and dies
- Abrasive products that have ground steel parts on your aluminum project. You should keep a set of abrasive discs, belts, and flap discs for use only on aluminum. Using discs that have ground aluminum to grind steel is fine.
Proper Abrasives and Cutting Tool Selection
What are the best abrasives for cutting and grinding aluminum?
You need to select abrasives suitable for grinding aluminum. The best abrasive grains to cut and grind aluminum alloys include aluminum oxide, silicon carbide, blends of aluminum oxide, silicon carbide, zirconia, and ceramic. Since most aluminum alloys are exceptionally soft, extremely wear-resistant zirconia and ceramic abrasive grains can be overkill. Loading of the coated abrasive surfaces is more of a problem than dulling of the abrasive grain tips.
Calcium stearate wax as well as grinding aid or supersize layers on a coated abrasive product can enhance the amount of metal removed or parts ground. Supersize provides a lubricating and cooling effect. The supersize also helps reduce the binding of aluminum chips to the cutting edges of the abrasive grains and the loading of the abrasive surface.
Always start with the finest grit size recommended or try even finer. The finer grit size reduces heating and discoloration on aluminum while reducing the work you must do later to remove scratches and create a beautiful aluminum finish.
Grease Sticks and Abrasive Cleaners
Two additional options to enhance aluminum sanding or grinding efficiency and prolong coated abrasive belt and disc life are grease sticks and abrasive cleaners.
Grease sticks are used to reduce loading when sanding wood or grinding aluminum and other soft, gummy metals – especially if the coated abrasive product does have a top layer of calcium stearate or grinding aid. The grease stick is applied to the surface of the moving coated abrasive disc or belt product. The grease provides a lubricant and non-stick action, so the aluminum swarfs cannot stick to abrasives as easily.
Abrasive cleaners are rubbery sticks used to remove loading on a coated abrasive surface. The abrasive cleaning stick is pressed into a moving coated abrasive belt or disc to clean the surface.
Saw Blades and Cutting Tools
Coarse toothed saw blades or blades with lower teeth per inch (TPI) number should clog or load less compared to finer toothed blades. There are saw blades designed specifically for cutting aluminum where the cutting edge geometry is optimized to eject or clear the aluminum chip to reduce or prevent loading.
Grease sticks are also useful in lubricant toothed saw blades and hole saws when cutting aluminum. Apply a coating to the blade after every 5 cuts.
What’s next and how can I get started?

Once you have decided what surface finish you or your customer wants on the aluminum project you are fabricating, then you can select and order abrasive products, so they will be within your hand’s reach when you have finished cutting, bending, forming, and welding.
Here is a good list of abrasive products for your aluminum grinding and finishing toolbox:
- Stainless Steel Wire Brushes – For weld joint pre-cleaning and preparation as well as post-weld cleaning and smut removal.
- Surface Conditioning Discs – For weld joint pre-cleaning and preparation as well as post-weld cleaning and smut removal
- Saw Blades and Aluminum Cutting Discs – Aluminum can be easily cut with reciprocating saw blades, hole saws, and abrasive cutting discs design for aluminum cutting.
- Half-round Aluminum Files – For deburring edges after cutting and shaping joints before welding.
- Stearated Flap Discs 60 grit Type T27 and Type T29– For flattening moderately concave weld beads, shaping parts, and removing oxide scale from welds backsides, forgings, and heat-treated parts, and blending.
- 4” x 4” Interleaf Flap Wheel Drum 120 and 240 grit – For smut removal, deburring, and generating a directional grain on aluminum surfaces.
- Finer Grit Abrasive Belts - For smut removal, deburring, and generating a directional grain on aluminum. A series of grits sizes are needed depending on the finish you want (Brushed – 150/180 grit, Satin 180/220 grit, Matte 240/280 grit)
Still Have Questions about Abrasives for Aluminum?
We tried to make this a comprehensive as we could for an aluminum abrasives guide, but if you still have any questions, we are available to assist. Our abrasives experts are available by phone, email, or chat to discuss your upcoming on ongoing projects.


